Effects of Relative Humidity on Ozone and Secondary Organic Aerosol Formation from the Photooxidation of Benzene and Ethylbenzene
组内消息 2013-11-07
SOA samples with heating were heated at 110°C for 15 min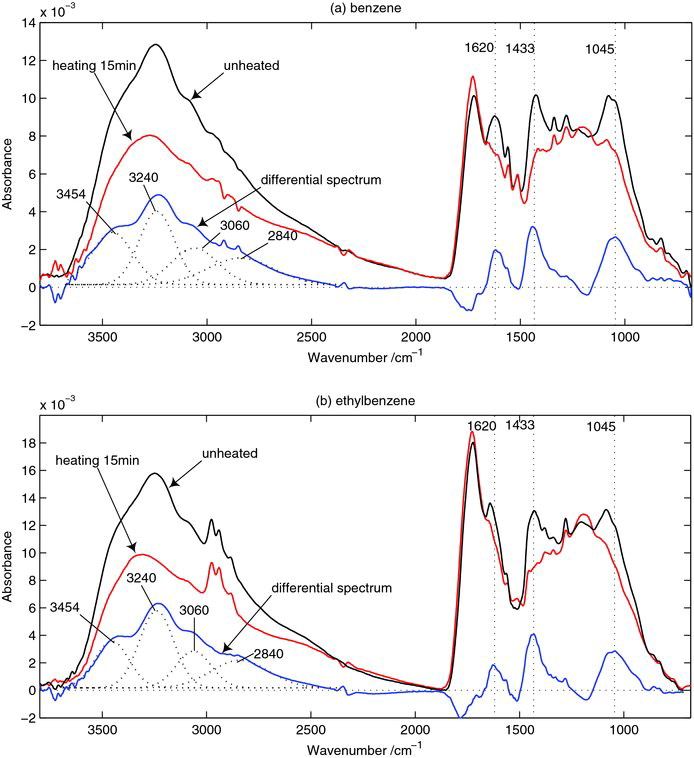
The formation of ozone and secondary organic aerosol (SOA) from benzene–NO x and ethylbenzene–NOx irradiations was investigated under different levels of relative humidity (RH) in a smog chamber. In benzene and ethylbenzene irradiations, the intensity of the bands of O‒H, C=O, C‒O, and C‒OH from SOA samples all greatly increased with increasing RH. The major substances in SOA were determined to be carboxylic acids and glyoxal hydrates. It was also found that SOA contained aromatic products, and NO2- and ONO2-containing products. The results show that the increase in RH can greatly reduce the maximum O3 by the transfer of NO2- and ONO2-containing products into the particle phase. During the process of evaporation, the lost substances from the collected SOA have similar structures for both benzene and ethylbenzene. This demonstrates that ethyl-containing substances are very stable and difficult to evaporate. For benzene, some of glyoxal hydrates were left to form C‒O‒C- and C˭O-containing species like hemiacetal and acetal after evaporation, whereas for ethylbenzene, glyoxal favored cross reactions with ethylglyoxal during evaporation. Only a few species in SOA were released into the gas phase during evaporation while a large part of SOA remained, which is mainly composed of carboxylic acid. It is concluded that the aqueous radical reactions and the hydration from glyoxal can be enhanced under high RH conditions, which can irreversibly enhance the formation of SOA from both benzene and ethylbenzene. 全文下载
Jia L, Xu Y F. Effects of Relative Humidity on Ozone and Secondary Organic Aerosol Formation from the Photooxidation of Benzene and Ethylbenzene. Aerosol Science and Technology, 2014, 48(1):1-12.
Related Posts: